Contamination can occur at any stage during the production of pharmaceuticals, posing significant health risks. This brief overview examines typical contamination sources in drug manufacturing, response strategies when contamination happens, and preventative measures.
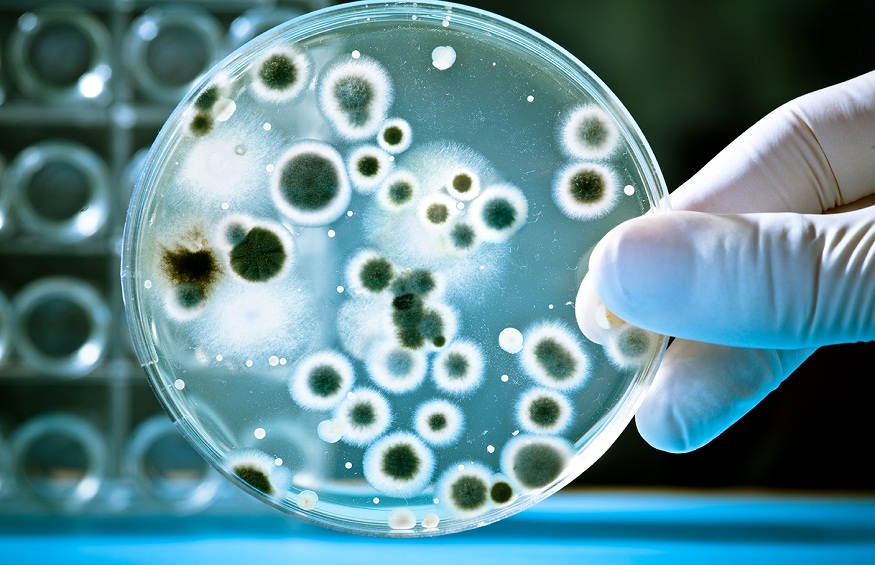
Contamination in pharmaceutical products can stem from a lack of adherence to strict microbiological procedures. Laboratories dealing with microbiology must be cautious, as workers are often in contact with chemical and biological substances that could be hazardous, including pathogens or radioactive materials.
Breakdowns in procedures can introduce contaminants into the drug production process. These may originate from poorly designed facilities or equipment, inadequate training of personnel, errors in storing materials, insufficient air filtration, or cross-contamination between products.
Immediate and thorough investigation is crucial when contamination is detected. Utilizing pharmaceutical and biopharmaceutical contaminant testing services aligns with industry standards and aids in pinpointing and rectifying the contamination source. Following identification and mitigation, it is critical to decontaminate and retest the area to confirm its sterility.
Prevention is better than a cure when it comes to contamination. Protocols should be revised and enhanced post-contamination, addressing any identified gaps, such as equipment defects, handling errors, or facility issues like inadequate ventilation. Continual refinement of prevention strategies can strengthen facility operations and employee practices.
For more detailed guidance on handling pharmaceutical contamination, the accompanying resource is a valuable tool.

Infographic created by BA Sciences, a leader in pharmaceutical testing innovation








+ There are no comments
Add yours